Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders
Molecular Autism volume 7, Article number: 49 (2016)
Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders
Maria Fiorentino 1 , Anna Sapone 2 , Stefania Senger 1 , Stephanie S Camhi 3 , Sarah M Kadzielski 4 , Timothy M Buie 4 , Deanna L Kelly 5 , Nicola Cascella 6 , Alessio Fasano 7
Affiliations
1Mucosal Immunology and Biology Research Center, Massachusetts General Hospital for Children, Boston, MA USA ; Department of Pediatrics, Harvard Medical School, Boston, MA USA.
2 Mucosal Immunology and Biology Research Center, Massachusetts General Hospital for Children, Boston, MA USA ; Department of Medicine, Celiac Center, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA USA.
3 Mucosal Immunology and Biology Research Center, Massachusetts General Hospital for Children, Boston, MA USA ; Center for Celiac Research and Division of Pediatric Gastroenterology and Nutrition, Massachusetts General Hospital for Children, Boston, MA USA.
4 Department of Pediatrics, Harvard Medical School, Boston, MA USA.
5 Maryland Psychiatric Research Center, University of Maryland School of Medicine, Baltimore, MD USA. 6 Neuropsychiatry Program, Sheppard Pratt Health System, Baltimore, MD USA.
7 Mucosal Immunology and Biology Research Center, Massachusetts General Hospital for Children, Boston, MA USA ; Center for Celiac Research and Division of Pediatric Gastroenterology and Nutrition, Massachusetts General Hospital for Children, Boston, MA USA ; Department of Pediatrics, Harvard Medical School, Boston, MA USA.
Abstract
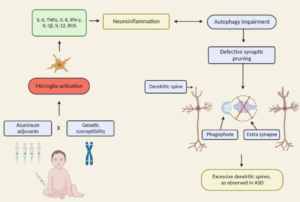
Background Autism spectrum disorders (ASD) are complex conditions whose pathogenesis may be attributed to gene–environment interactions. There are no definitive mechanisms explaining how environmental triggers can lead to ASD although the involvement of inflammation and immunity has been suggested. Inappropriate antigen trafficking through an impaired intestinal barrier, followed by passage of these antigens or immune-activated complexes through a permissive blood–brain barrier (BBB), can be part of the chain of events leading to these disorders. Our goal was to investigate whether an altered BBB and gut permeability is part of the pathophysiology of ASD. Methods Postmortem cerebral cortex and cerebellum tissues from ASD, schizophrenia (SCZ), and healthy subjects (HC) and duodenal biopsies from ASD and HC were analyzed for gene and protein expression profiles. Tight junctions and other key molecules associated with the neurovascular unit integrity and function and neuroinflammation were investigated. Results Claudin (CLDN)-5 and -12 were increased in the ASD cortex and cerebellum. CLDN-3, tricellulin, and MMP-9 were higher in the ASD cortex. IL-8, tPA, and IBA-1 were downregulated in SCZ cortex; IL-1b was increased in the SCZ cerebellum. Differences between SCZ and ASD were observed for most of the genes analyzed in both brain areas. CLDN-5 protein was increased in ASD cortex and cerebellum, while CLDN-12 appeared reduced in both ASD and SCZ cortexes. In the intestine, 75% of the ASD samples analyzed had reduced expression of barrier-forming TJ components (CLDN-1, OCLN, TRIC), whereas 66% had increased pore-forming CLDNs (CLDN-2, -10, -15) compared to controls. Conclusions In the ASD brain, there is an altered expression of genes associated with BBB integrity coupled with increased neuroinflammation and possibly impaired gut barrier integrity. While these findings seem to be specific for ASD, the possibility of more distinct SCZ subgroups should be explored with additional studies.