Neuroinflammation and autism: toward mechanisms and treatments
Neuropsychopharmacology. 2013 Jan;38(1):241-2. doi: 10.1038/npp.2012.174.
Neuroinflammation and autism: toward mechanisms and treatments
Christopher J McDougle 1 , William A Carlezon Jr
1 Department of Psychiatry, Massachusetts General Hospital and Mass General Hospital for Children, Boston, MA, USA.
2 Harvard Medical School, Boston, MA, USA; 3 Department of Psychiatry, McLean Hospital, Belmont, MA, USA
Autism spectrum disorders (ASDs) were originally described by Kanner (1943). The relatively consistent clinical phenotype will likely be shown to comprise numerous etiologic subtypes. Approximately 10% of ASD cases are linked to disorders of genetic etiology, such as Fragile X syndrome, tuberous sclerosis, and Rett disorder. The majority of cases, however, remain idiopathic.
A role for immunological involvement in ASDs has long been hypothesized. Kanner did not comment on this in his initial descriptions, but a detailed review of the original 11 cases reveals important observations. One patient was ‘kept in bed often because of colds, bronchitis, chickenpox, streptococcus infection, impetigo and rheumatic fever’. Another was ‘given anterior pituitary and thyroid preparations and her father, aged 36 years, was one of those chronically thin persons, nervous energy readily expended’, suggesting hyperthyroidism.
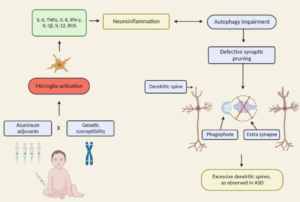
In our clinical work and review of the literature, we have been impressed by the possible role of autoimmune disorders as influencing the pathophysiology of a distinct, objectively defined etiologic subtype of ASDs. Money et al (1971) published what is widely accepted as the first connection between autism and autoimmune disorders. They described a family in which the youngest child had multiple diagnoses, including autism, Addison’s disease, moniliasis, and diabetes mellitus. The next older brother had hypoparathyroidism, Addison’s disease, moniliasis, and alopecia totalis. The oldest son was symptom-free. The mother had ulcerative colitis, the father had ‘chronic athlete’s foot’, and a paternal uncle had diabetes mellitus. Consistent with these observations, we showed that first- and second-degree relatives of children with an ASD have a higher number of autoimmune disorders than family members of healthy children (Sweeten et al, 2003). In a recent post-mortem study of 13 males with autism and 9 control cases, microglia appeared markedly activated in 5 of 13 cases with autism, including 2 of 3 under the age of 6 years, and marginally activated in an additional 4 of 13 cases (Morgan et al, 2010), suggesting ongoing inflammatory processes in brain.
Observations in humans are supported by experiments in laboratory animals. As one example, Martin et al (2008) exposed pregnant rhesus monkeys to human IgG collected from mothers of children diagnosed with ASDs, while controls received IgG collected from mothers of normally developing children. Those offspring that were gestationally exposed to IgG class antibodies from mothers of children with ASDs consistently demonstrated increases in stereotypies and hyperactivity. These findings suggest that some ASD-like behaviors can be triggered by environmental (non-genetic) manipulations.
The notion that environmental factors contribute to ASD prevalence continues to evolve. Once-influential theories suggesting links among exposure to vaccines containing attenuated virus or toxins, conditions such as inflammatory bowel disease, and ASDs have fallen from favor since the retraction of a key study (Wakefield et al, 1998). It is important to emphasize, however, that the major reason for retraction was poor scientific method rather than theoretical flaws. Although ASDs are currently within the realm of psychiatrists and neurologists, it is becoming clear that at least some subtypes represent whole-body disorders, offering exciting new possibilities for therapy.