Does acute exposure to thimerosal, an organic mercury compound, affect the mitochondrial function of an infant model?
Journal of Trace Elements in Medicine and Biology
Volume 83, May 2024
Marcos V.S. Sales a, Ellen dos Santos Silva Barros a, Rafael D.S. Azevedo b, Francisco A.S. Cunha c, Josué Carinhanha C. Santos a1, Ana C.R. Leite a2a
Universidade Federal de Alagoas (UFAL),
Campus A. C. Simões, 57072-900 Maceió,
Alagoas, BrazilbUniversidade de Pernambuco (UPE),
Campus Garanhuns, 55294-902 São José, Pernambuco,
BrazilcInstituto de Química,
Universidade Federal da Bahia (UFBA),
Campus Ondina, 40170-115 Salvador, Bahia, Brazil
Abstract
Background
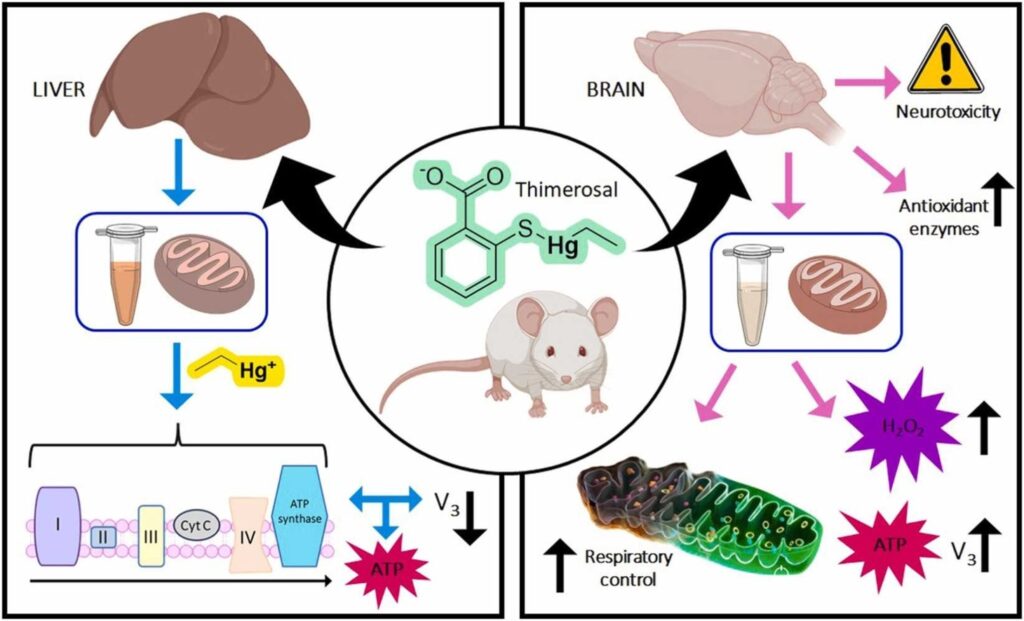
Thimerosal (TM) is a toxic, organometallic mercury compound (which releases ethyl-mercury-containing compounds in aqueous solutions) used as a preservative in vaccines. Mitochondria are organelle which are highly vulnerable to many chemical compounds, including mercury (Hg) and its derivatives.
Method
Wistar rats (at 21 days of age) were used to model a child’s TM exposure following childhood vaccination, divided in two groups: TM exposed (20 μg/kg/day) and unexposed controls (saline solution), both for 24 h. Atomic Fluorescence Spectrometry was used to quantify the amounts of mercury in tissues. The electron transport chain (ETC) from isolated mitochondria was evaluated using an oxygen electrode. The mitochondrial membrane potential and H2O2 production were analyzed using selective fluorescence probes. The activity of some enzymes (SOD, CAT, GPx, and AChE) and secondary markers of oxidative stress (GSH, GSSG, total free thiol) were also examined in tissues.
Results
Hg accumulation in the brain and liver was higher in exposed animals when compared to the control. Liver-isolated mitochondria showed that TM improved respiratory control by 23%; however, states 3 and 4 of the ETC presented a decrease of 16% and 37%, respectively. Furthermore, brain-isolated mitochondria presented an improvement of 61% in respiratory control. Brain enzyme activities were significantly impacted in TM-exposed rats compared to unexposed rats as follows: decreases in SOD (32%) and AChE (42%) and increases in GPx (79%) and CAT (100%). GPx enzyme activity in the liver was significantly increased (37%). Among secondary oxidative stress markers, the brain’s total reduced thiol (SH) concentration was significantly increased (41%).
Conclusion
Acute TM treatment exposure in a Wistar rat model mimicking TM exposure in an infant following childhood vaccination significantly damaged brain bioenergetic pathways. This study supports the ability of TM exposure to preferentially damage the nervous system.