Exceprts:
We also discuss evidence implicating oxidative stress, neuroglial activation and neuroimmunity in autism.
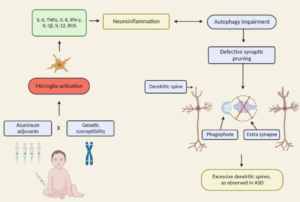
“Oxidative stress is another possible cause of Purkinje cell loss and other neuroanatomical changes described in autistic brains (reviewed in (37, 113)). Oxidative stress occurs when the levels of reactive oxygen species exceed the antioxidant capacities of a cell, often leading to cell death. Because of its very high oxygen demands and limited anti-oxidant capacity, the brain is thought to be relatively vulnerable to oxidative stress (111). Several studies have shown decreased levels of antioxidants such as superoxide dismutase, transferrin and ceruloplasmin in the blood or serum of patients with ASD (38, 108, 222). Significant elevations in biomarker profiles indicating increased oxidative stress, such as increased lipid peroxidation, have also been documented in autism (38, 107, 229).Interestingly, in one report the alterations in antioxidant proteins were linked specifically to regressive autism, suggesting a postnatal environmental effect (38). Polymorphisms in metabolic pathway genes may contribute to the increased oxidative stress in autism (108). Advanced glycationend products have also been reported to be elevated in both the brain tissue and serum of autistic patients, a change which can also lead to increased oxidative damage (23,110).”
More