Conclusions
In the ASD brain, there is an altered expression of genes associated with BBB integrity coupled with increased neuroinflammation and possibly impaired gut barrier integrity.
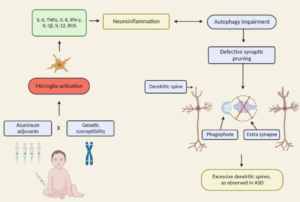
Neuroinflammation
Excerpt: “Oxidative stress, brain inflammation, and microgliosis have been much documented in association with toxic exposures including various heavy metals…the awareness that the brain as well as medical conditions of children with autism may be conditioned by chronic biomedical abnormalities such as inflammation opens the possibility that meaningful biomedical interventions may be possible well past the window of maximal neuroplasticity in early childhood because the basis for assuming that all deficits can be attributed to fixed early developmental alterations in neural architecture has now been undermined.”
Excerpt:
“Levels of serum neurokinin A and BHg were measured in 84 children with ASD, aged between 3 and 10 years, and 84 healthy-matched children. There was a positive linear relationship between the Childhood Autism Rating Scale (CARS) and both serum neurokinin A and BHg. ASD children had significantly higher levels of serum neurokinin A than healthy controls (P < 0.001). Increased levels of serum neurokinin A and BHg were respectively found in 54.8 % and 42.9 % of the two groups. There was significant and positive linear relationship between levels of serum neurokinin A and BHg in children with moderate and severe ASD, but not in healthy control children. It was found that 78.3 % of the ASD patients with increased serum levels of neurokinin A had elevated BHg levels (P < 0.001). Neuroinflammation, with increased levels of neurokinin A, is seen in some children with ASD, and may be caused by elevated BHg levels. Further research is recommended to determine the pathogenic role of increased levels of serum neurokinin A and BHg in ASD. The therapeutic role of tachykinin receptor antagonists, a potential new class of anti-inflammatory medications, and Hg chelators, should also be studied in ASD."
Excerpt: “Taken together, the results suggest a close link between oxidative stress neuroinflamation and degeneration in aluminium-fluoride toxicity.”
Abstract
A role for immunological involvement in autism spectrum disorder (ASD) has long been hypothesized. This review includes four sections describing (1) evidence for a relationship between familial autoimmune disorders and ASD; (2) results from post-mortem and neuroimaging studies that investigated aspects of neuroinflammation in ASD; (3) findings from animal model work in ASD involving inflammatory processes; and (4) outcomes from trials of anti-inflammatory/immune-modulating drugs in ASD that have appeared in the literature. Following each section, ideas are provided for future research, suggesting paths forward in the continuing effort to define the role of immune factors and inflammation in the pathophysiology of a subtype of ASD
Abstract
Autism spectrum disorders (ASDs) are complex, heterogeneous disorders caused by an interaction between genetic vulnerability and environmental factors. In an effort to better target the underlying roots of ASD for diagnosis and treatment, efforts to identify reliable biomarkers in genetics, neuroimaging, gene expression, and measures of the body’s metabolism are growing. For this article, we review the published studies of potential biomarkers in autism and conclude that while there is increasing promise of finding biomarkers that can help us target treatment, there are none with enough evidence to support routine clinical use unless medical illness is suspected. Promising biomarkers include those for mitochondrial function, oxidative stress, and immune function. Genetic clusters are also suggesting the potential for useful biomarkers.
Excerpt: “A recent review assessed the research on physiological abnormalities associated with ASD (44). The authors identified four main mechanisms that have been increasingly studied during the past decade: immunologic/inflammation, oxidative stress, environmental toxicants, and mitochondrial abnormalities. In addition, there is accumulating research on the lipid, GI systems, microglial activation, and the microbiome, and how these can also contribute to generating biomarkers associated with ASD (45, 46).
Pathways are interconnected with a defect in one likely leading to dysfunction in others. Many metabolic disorders can lead to endpoints such as impaired methylation, sulfuration, and detoxification pathways and nutritional deficiencies. Mitochondrial dysfunction, environmental risk factors, metabolic imbalances, and genetic susceptibility can all lead to oxidative stress (47), which in turn leads to inflammation, damaged cell membranes, autoimmunity (48), impaired methylation (49), cell death (48), and neurological deficits (50). The brain is highly vulnerable to oxidative stress (51), particularly in children (52) during the early part of development (47). As environmental events and metabolic imbalances affect oxidative stress and methylation, they also can affect the expression of genes.”
Excerpts:
“In our clinical work and review of the literature, we have been impressed by the possible role of autoimmune disorders as influencing the pathophysiology of a distinct, objectively defined etiologic subtype of ASDs.”
“The notion that environmental factors contribute to ASD prevalence continues to evolve. Once-influential theories suggesting links among exposure to vaccines containing attenuated virus or toxins, conditions such as inflammatory bowel disease, and ASDs have fallen from favor since the retraction of a key study (Wakefield et al, 1998). It is important to emphasize, however, that the major reason for retraction was poor scientific method rather than theoretical flaws. Although ASDs are currently within the realm of psychiatrists and neurologists, it is becoming clear that at least some subtypes represent whole-body disorders, offering exciting new possibilities for therapy.”
Excerpts:
“The current literature suggests an imbalance of oxidative and anti-oxidative stress systems in autism. Glutathione is involved in neuro-protection against oxidative stress and neuro-inflammation in autism by improving the anti-oxidative stress system. Decreasing the oxidative stress might be a potential treatment for autism.”
Excerpt:
“When assessing adjuvant toxicity in children, several key points ought to be considered: (i) infants and children should not be viewed as “small adults” with regard to toxicological risk as their unique physiology makes them much more vulnerable to toxic insults; (ii) in adult humans Al vaccine adjuvants have been linked to a variety of serious autoimmune and inflammatory conditions (i.e., “ASIA”), yet children are regularly exposed to much higher amounts of Al from vaccines than adults; (iii) it is often assumed that peripheral immune responses do not affect brain function. However, it is now clearly established that there is a bidirectional neuro-immune cross-talk that plays crucial roles in immunoregulation as well as brain function. In turn, perturbations of the neuro-immune axis have been demonstrated in many autoimmune diseases encompassed in “ASIA” and are thought to be driven by a hyperactive immune response; and (iv) the same components of the neuro-immune axis that play key roles in brain development and immune function are heavily targeted by Al adjuvants.”
Excerpt:
“We found that abnormal brain enlargement was most commonly found in boys with regressive autism. Brain size in boys without regression did not differ from controls.”
“These results suggest that there may be distinct neural phenotypes associated with different onsets of autism.”