Excerpt:
“The results support the hypothesis that Hg sensitivity may be a heritable/genetic risk factor for ASD.”
Thimerosal
Excerpt:
“These data document that early postnatal THIM administration causes lasting neurobehavioral impairments and neurochemical alterations in the brain, dependent on dose and sex. If similar changes occur in THIM/mercurial-exposed children, they could contribute do neurodevelopmental disorders.”
Abstract
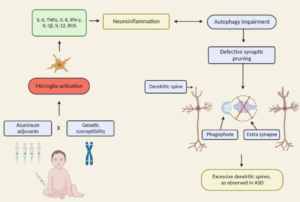
There is a need to interpret neurotoxic studies to help deal with uncertainties surrounding pregnant mothers, newborns and young children who must receive repeated doses of Thimerosal-containing vaccines (TCVs). This review integrates information derived from emerging experimental studies (in vitro and in vivo) of low-dose Thimerosal (sodium ethyl mercury thiosalicylate). Major databases (PubMed and Web-of-science) were searched for in vitro and in vivo experimental studies that addressed the effects of low-dose Thimerosal (or ethylmercury) on neural tissues and animal behaviour. Information extracted from studies indicates that: (a) activity of low doses of Thimerosal against isolated human and animal brain cells was found in all studies and is consistent with Hg neurotoxicity; (b) the neurotoxic effect of ethylmercury has not been studied with co-occurring adjuvant-Al in TCVs; (c) animal studies have shown that exposure to Thimerosal-Hg can lead to accumulation of inorganic Hg in brain, and that (d) doses relevant to TCV exposure possess the potential to affect human neuro-development. Thimerosal at concentrations relevant for infants’ exposure (in vaccines) is toxic to cultured human-brain cells and to laboratory animals. The persisting use of TCV (in developing countries) is counterintuitive to global efforts to lower Hg exposure and to ban Hg in medical products; its continued use in TCV requires evaluation of a sufficiently nontoxic level of ethylmercury compatible with repeated exposure (co-occurring with adjuvant-Al) during early life.
Excerpt:
“These findings document neurotoxic effects of thimerosal, at doses equivalent to those used in infant vaccines or higher, in developing rat brain, suggesting likely involvement of this mercurial in neurodevelopmental disorders.”
Excerpt:
“Boys vaccinated as neonates had threefold greater odds for autism diagnosis compared to boys never vaccinated or vaccinated after the first month of life.”
Abstract
Mercury toxicity is a highly interesting topic in biomedicine due to the severe endpoints and treatment limitations. Selenite serves as an antagonist of mercury toxicity, but the molecular mechanism of detoxification is not clear. Inhibition of the selenoenzyme thioredoxin reductase (TrxR) is a suggested mechanism of toxicity. Here, we demonstrated enhanced inhibition of activity by inorganic and organic mercury compounds in NADPH-reduced TrxR, consistent with binding of mercury also to the active site selenolthiol. On treatment with 5 μM selenite and NADPH, TrxR inactivated by HgCl(2) displayed almost full recovery of activity. Structural analysis indicated that mercury was complexed with TrxR, but enzyme-generated selenide removed mercury as mercury selenide, regenerating the active site selenocysteine and cysteine residues required for activity. The antagonistic effects on TrxR inhibition were extended to endogenous antioxidants, such as GSH, and clinically used exogenous chelating agents BAL, DMPS, DMSA, and α-lipoic acid. Consistent with the in vitro results, recovery of TrxR activity and cell viability by selenite was observed in HgCl(2)-treated HEK 293 cells. These results stress the role of TrxR as a target of mercurials and provide the mechanism of selenite as a detoxification agent for mercury poisoning.
Excerpts:
“These data document that exposure to thimerosal during early postnatal life produces lasting alterations in the densities of brain opioid receptors along with other neuropathological changes, which may disturb brain development.”
Conclusions: HgCl2 stimulates VEGF and IL-6 release from human mast cells. This phenomenon could disrupt the blood-brain-barrier and permit brain inflammation. As a result, the findings of the present study provide a biological mechanism for how low levels of mercury may contribute to ASD pathogenesis.
Excerpt:
“We argue that scientific research does not support rejecting the link between the neurodevelopmental disorder of autism and toxic exposures.”
Abstract
BACKGROUND: Increased urinary concentrations of pentacarboxyl-, precopro- and copro-porphyrins have been associated with prolonged mercury (Hg) exposure in adults, and comparable increases have been attributed to Hg exposure in children with autism (AU).
OBJECTIVES: This study was designed to measure and compare urinary porphyrin concentrations in neurotypical (NT) children and same-age children with autism, and to examine the association between porphyrin levels and past or current Hg exposure in children with autism.
METHODS: This exploratory study enrolled 278 children 2-12 years of age. We evaluated three groups: AU, pervasive developmental disorder-not otherwise specified (PDD-NOS), and NT. Mothers/caregivers provided information at enrollment regarding medical, dental, and dietary exposures. Urine samples from all children were acquired for analyses of porphyrin, creatinine, and Hg. Differences between groups for mean porphyrin and Hg levels were evaluated. Logistic regression analysis was conducted to determine whether porphyrin levels were associated with increased risk of autism.
RESULTS: Mean urinary porphyrin concentrations are naturally high in young children and decline by as much as 2.5-fold between 2 and 12 years of age. Elevated copro- (p < 0.009), hexacarboxyl- (p < 0.01) and pentacarboxyl- (p < 0.001) porphyrin concentrations were significantly associated with AU but not with PDD-NOS. No differences were found between NT and AU in urinary Hg levels or in past Hg exposure as determined by fish consumption, number of dental amalgam fillings, or vaccines received. CONCLUSIONS:These findings identify disordered porphyrin metabolism as a salient characteristic of autism. Hg exposures were comparable between diagnostic groups, and a porphyrin pattern consistent with that seen in Hg-exposed adults was not apparent.