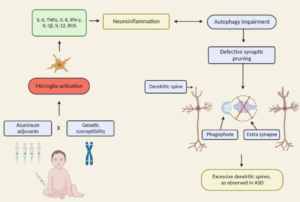
Excerpt:
“Environmental mercury is neurotoxic at doses well below the current reference levels considered to be safe, with evidence of neurotoxicity in children exposed to environmental sources including fish consumption and ethylmercury-containing vaccines. Possible neurotoxic mechanisms of mercury include direct effects on sulfhydryl groups, pericytes and cerebral endothelial cells, accumulation within astrocytes, microglial activation, induction of chronic oxidative stress, activation of immune-inflammatory pathways and impairment of mitochondrial functioning. (Epi-)genetic factors which may increase susceptibility to the toxic effects of mercury in ASD include the following: a greater propensity of males to the long-term neurotoxic effects of postnatal exposure and genetic polymorphisms in glutathione transferases and other glutathione-related genes and in selenoproteins. Furthermore, immune and inflammatory responses to immunisations with mercury-containing adjuvants are strongly influenced by polymorphisms in the human leukocyte antigen (HLA) region and by genes encoding effector proteins such as cytokines and pattern recognition receptors. Some epidemiological studies investigating a possible relationship between high environmental exposure to methylmercury and impaired neurodevelopment have reported a positive dose-dependent effect.”
Toxicants
“Conclusion
We found that blood mercury levels at late pregnancy and early childhood were associated with more autistic behaviors in children at 5 years of age. Further study on the long-term effects of mercury exposure is recommended.”
Excerpts:
“…several large scale epidemiological studies have recently linked prenatal air pollution exposure with an increased risk of neurodevelopmental disorders such as autism spectrum disorder (ASD).”
“We have demonstrated that prenatal exposure to DEP in mice, i.e., to the pregnant dams throughout gestation, results in a persistent vulnerability to behavioral deficits in adult offspring, especially in males, which is intriguing given the greater incidence of ASD in males to females (∼4:1).”
“DEP exposure increased inflammatory cytokine protein and altered the morphology of microglia, consistent with activation or a delay in maturation, only within the embryonic brains of male mice…”
“Consistent with this hypothesis, we found increased microglial-neuronal interactions in male offspring that received DEP compared to all other groups. Taken together, these data suggest a mechanism by which prenatal exposure to environmental toxins may affect microglial development and long-term function, and thereby contribute to the risk of neurodevelopmental disorders.”
Abstract
Environmental factors have been implicated in the etiology of autism spectrum disorder (ASD); however, the role of heavy metals has not been fully defined. This study investigated whether blood levels of mercury, arsenic, cadmium, and lead of children with ASD significantly differ from those of age- and sex-matched controls. One hundred eighty unrelated children with ASD and 184 healthy controls were recruited. Data showed that the children with ASD had significantly (p < 0.001) higher levels of mercury and arsenic and a lower level of cadmium. The levels of lead did not differ significantly between the groups. The results of this study are consistent with numerous previous studies, supporting an important role for heavy metal exposure, particularly mercury, in the etiology of ASD. It is desirable to continue future research into the relationship between ASD and heavy metal exposure.
Abstract
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder that affects social, communication, and behavioral development. Recent evidence supported but also questioned the hypothetical role of compounds containing mercury (Hg) as contributors to the development of ASD. Specific alterations in the urinary excretion of porphyrin-containing ring catabolites have been associated with exposure to Hg in ASD patients. In the present study, the level of urinary porphyrins, as biomarkers of Hg toxicity in children with ASD, was evaluated, and its correlation with severity of the autistic behavior further explored. A total of 100 children was enrolled in the present study. They were classified into three groups: children with ASD (40), healthy controls (40), and healthy siblings of the ASD children (20). Children with ASD were diagnosed using DSM-IV-TR, ADI-R, and CARS tests. Urinary porphyrins were evaluated within the three groups using high-performance liquid chromatography (HPLC), after plasma evaluation of mercury (Hg) and lead (Pb) in the same groups. Results showed that children with ASD had significantly higher levels of Hg, Pb, and the porphyrins pentacarboxyporphyrin, coproporphyrin, precoproporphyrin, uroporphyrins, and hexacarboxyporphyrin compared to healthy controls and healthy siblings of the ASD children. However, there was no significant statistical difference in the level of heptacarboxyporphyrin among the three groups, while a significant positive correlation between the levels of coproporphyrin and precoproporphyrin and autism severity was observed. Mothers of ASD children showed a higher percentage of dental amalgam restorations compared to the mothers of healthy controls suggesting that high Hg levels in children with ASD may relate to the increased exposure to Hg from maternal dental amalgam during pregnancy and lactation. The results showed that the ASD children in the present study had increased blood Hg and Pb levels compared with healthy control children indicating that disordered porphyrin metabolism might interfere with the pathology associated with the autistic neurologic phenotype. The present study indicates that coproporphyrin and precoproporhyrin may be utilized as possible biomarkers for heavy metal exposure and autism severity in children with ASD.
Excerpt:
“We have reanalyzed the data set originally reported by Ip et al. in 2004 and have found that the original p value was in error and that a significant relation does exist between the blood levels of mercury and diagnosis of an autism spectrum disorder. Moreover, the hair sample analysis results offer some support for the idea that persons with autism may be less efficient and more variable at eliminating mercury from the blood.”
Excerpt: “Oxidative stress, brain inflammation, and microgliosis have been much documented in association with toxic exposures including various heavy metals…the awareness that the brain as well as medical conditions of children with autism may be conditioned by chronic biomedical abnormalities such as inflammation opens the possibility that meaningful biomedical interventions may be possible well past the window of maximal neuroplasticity in early childhood because the basis for assuming that all deficits can be attributed to fixed early developmental alterations in neural architecture has now been undermined.”
Excerpt:
“Levels of serum neurokinin A and BHg were measured in 84 children with ASD, aged between 3 and 10 years, and 84 healthy-matched children. There was a positive linear relationship between the Childhood Autism Rating Scale (CARS) and both serum neurokinin A and BHg. ASD children had significantly higher levels of serum neurokinin A than healthy controls (P < 0.001). Increased levels of serum neurokinin A and BHg were respectively found in 54.8 % and 42.9 % of the two groups. There was significant and positive linear relationship between levels of serum neurokinin A and BHg in children with moderate and severe ASD, but not in healthy control children. It was found that 78.3 % of the ASD patients with increased serum levels of neurokinin A had elevated BHg levels (P < 0.001). Neuroinflammation, with increased levels of neurokinin A, is seen in some children with ASD, and may be caused by elevated BHg levels. Further research is recommended to determine the pathogenic role of increased levels of serum neurokinin A and BHg in ASD. The therapeutic role of tachykinin receptor antagonists, a potential new class of anti-inflammatory medications, and Hg chelators, should also be studied in ASD."
Excerpts:
“The difference in manifested toxicity of MeHg and EtHg are likely the result of the differences in exposure, metabolism, and elimination from the body, rather than differences in mechanisms of action between the two.”
“Summary and Conclusions
There are many commonalities/similarities in the mechanisms of toxic action of methylmercury and ethylmercury (from thimerosal)… Evidence for the similarity of the various mechanisms of toxicity include the following:
• Both MeHg and EtHg bind to the amino acid cysteine (Clarkson 1995; Wu et al. 2008)…
• Both decrease glutathione activity, thus providing less protection from the oxidative stress caused by MeHg and EtHg (Carocci et al. 2014; Ndountse and Chan (2008); Choi et al. 1996; Franco et al. 2006; Mori et al. 2007; Muller et al. 2001; Ndountse and Chan 2008; Wu et al. 2008)…
• Both disrupt glutamate homeostasis (Farina et al. 2003a, b; Manfroi et al. 2004; Mutkus et al. 2005; Yin et al. 2007).
• Both cause oxidative stress/creation of ROS (Dreiem and Seegal 2007; Garg and Chang 2006; Myhre et al. 2003; Sharpe et al. 2012; Yin et al. 2007)…
• Both cause effects on receptor binding/neurotransmitter release involving one or more transmitters (Basu et al. 2008; Coccini et al. 2000; Cooper et al. 2003; Fonfria et al. 2001; Ida-Eto et al. 2011; Ndountse and Chan 2008; Yuan and Atchison 2003).
• Both cause DNA damage or impair DNA synthesis (Burke et al. 2006; Sharpe et al. 2012; Wu et al. 2008).”
Abstract
Prenatal and perinatal exposures to air pollutants have been shown to adversely affect birth outcomes in offspring and may contribute to prevalence of autism spectrum disorder (ASD). For this ecologic study, we evaluated the association between ASD prevalence, at the census tract level, and proximity of tract centroids to the closest industrial facilities releasing arsenic, lead or mercury during the 1990s. We used 2000 to 2008 surveillance data from five sites of the Autism and Developmental Disabilities Monitoring (ADDM) network and 2000 census data to estimate prevalence. Multi-level negative binomial regression models were used to test associations between ASD prevalence and proximity to industrial facilities in existence from 1991 to 1999 according to the US Environmental Protection Agency Toxics Release Inventory (USEPA-TRI). Data for 2489 census tracts showed that after adjustment for demographic and socio-economic area-based characteristics, ASD prevalence was higher in census tracts located in the closest 10th percentile compared of distance to those in the furthest 50th percentile (adjusted RR=1.27, 95% CI: (1.00, 1.61), P=0.049). The findings observed in this study are suggestive of the association between urban residential proximity to industrial facilities emitting air pollutants and higher ASD prevalence.